After almost a decade of effort, scientists at RIKEN have determined the structure of a key transporter protein that helps plants gather iron from soil. This finding could guide the development of new high-potency fertilizers that will help plants extract iron from iron-deficient soils.
Roughly a third of all land globally is alkaline because its soil contains large quantities of the alkaline salt, calcium carbonate. Iron does not dissolve well in these alkaline soils, and the resulting iron deficiency can severely restrict plant growth.
“Iron uptake from the soil is not easy,” says Atsushi Yamagata of the RIKEN Center for Biosystems Dynamics Research.
However, common grasses, including wheat and barley, have evolved a unique strategy to capture iron. They release compounds called phytosiderophores that are released into the soil, where they bind with iron and form a complex that the plants can absorb through their roots.
The phytosiderophores are compounds known as mugineic acids. While carrying their cargo of iron they are reabsorbed into plant cells by a transporter protein in cell membranes. But much is still unknown about the molecular mechanism of this process.
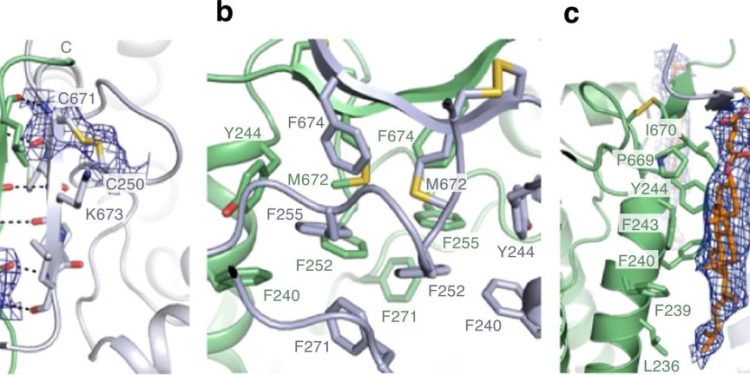
Now, Yamagata and his co-workers have determined the structure of the transporter protein for the first time.
“We have solved the structure of the transporter protein—both in the unbound state and when combined with an iron-carrying phytosiderophore,” says Yamagata. This is critical because it helps the researchers understand the fine molecular details of how the iron-containing complex interacts with the transporter to be carried into cells.
The RIKEN team had been trying to determine the transporter protein’s structure for nearly ten years. “We couldn’t even obtain the crystals needed for analysis by X-ray crystallography,” says Yamagata. The breakthrough came with recent advances in a technique called cryo-electron microscopy, which revealed the structures by firing electrons at frozen samples of the protein.
This research is now guiding work to develop derivatives of mugineic acids, which the team believe could become a new generation of highly effective fertilizers for alkaline soils.
“One synthetic derivative, developed by our collaborator Kosuke Namba of Tokushima University, can improve plant growth better than the natural compound at only around a thousandth of the cost,” says Yamagata. Called proline-2′-deoxymugineic acid (PDMA), the derivative is stable for a month in soil, compared to only a day for the natural compound.
Namba is now working with a Japanese manufacturer to scale up production of PDMA for commercial use as an agricultural fertilizer.